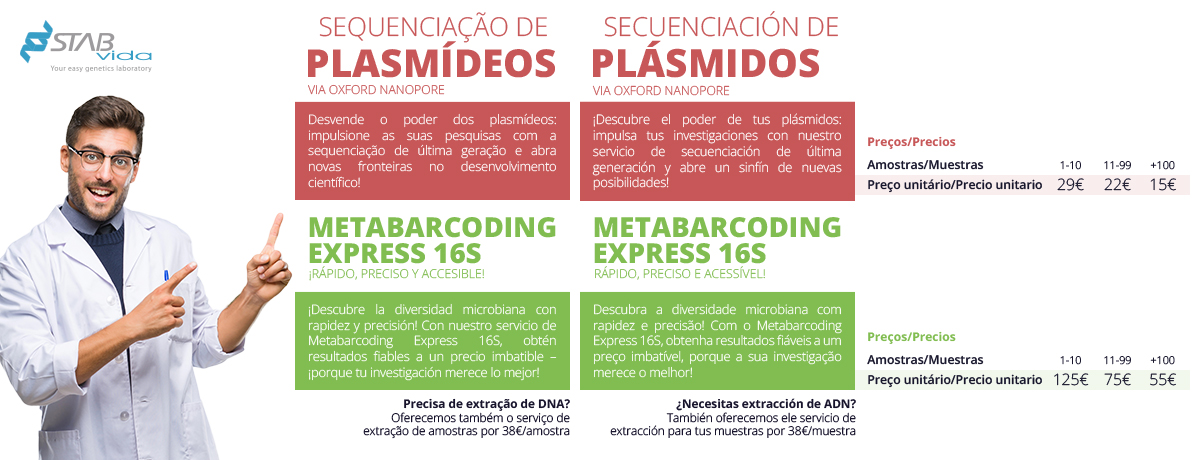
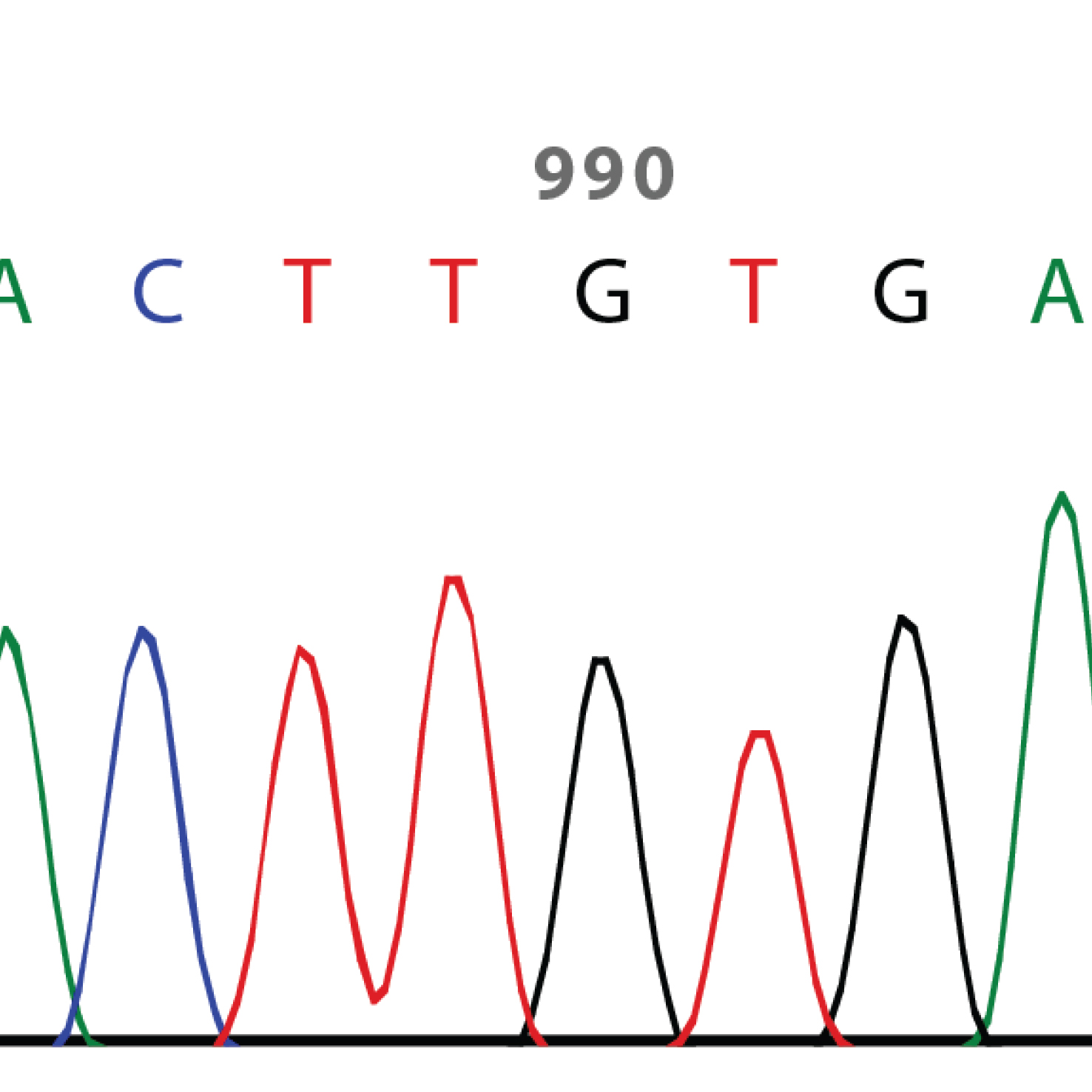
STAB VIDA is strategically located in the research and technology park of Madan Parque - FCT / UNL, with administrative and financial facilities, fully equipped laboratories with ISO9001:2015 and ISO13485:2018 certifications and a team specialized with more than 20 years of experience in the areas of genetics and genomics using PCR and nucleic acid sequencing technologies.
STAB VIDA, created in 2001, is a Portuguese SME in the field of biotechnology made up of 30 employees and an exclusive line of genetics and genomics products and services. Currently, its commercial territory covers Portugal, Spain, the United Kingdom, Italy, South America (Brazil, Chile and Uruguay) and Africa (Angola and Nigeria). The STAB VIDA team is made up of a CEO, CFO, COO, Project Manager, laboratory and manufacturing technicians, researchers and a commercial team.
STAB VIDA also has extensive experience in participating and coordinating national and European projects (FP6, FP7 and H2020). Special mention should be made of the participation of STAB VIDA in the EuroGentest NoE FP6 project as it allowed the entire genetic testing process to be harmonized, from sampling to processing, across Europe, which will be extremely important for the implementation of the easierNGS project and coordination of the Pulmagene, LungCard FP7 and LungCARD RISE projects, specifically in the design of the lung cancer pharmacogenomics NGS panel.



 Français
Français  Español
Español  Português
Português  Nederlands
Nederlands  Deutsch
Deutsch