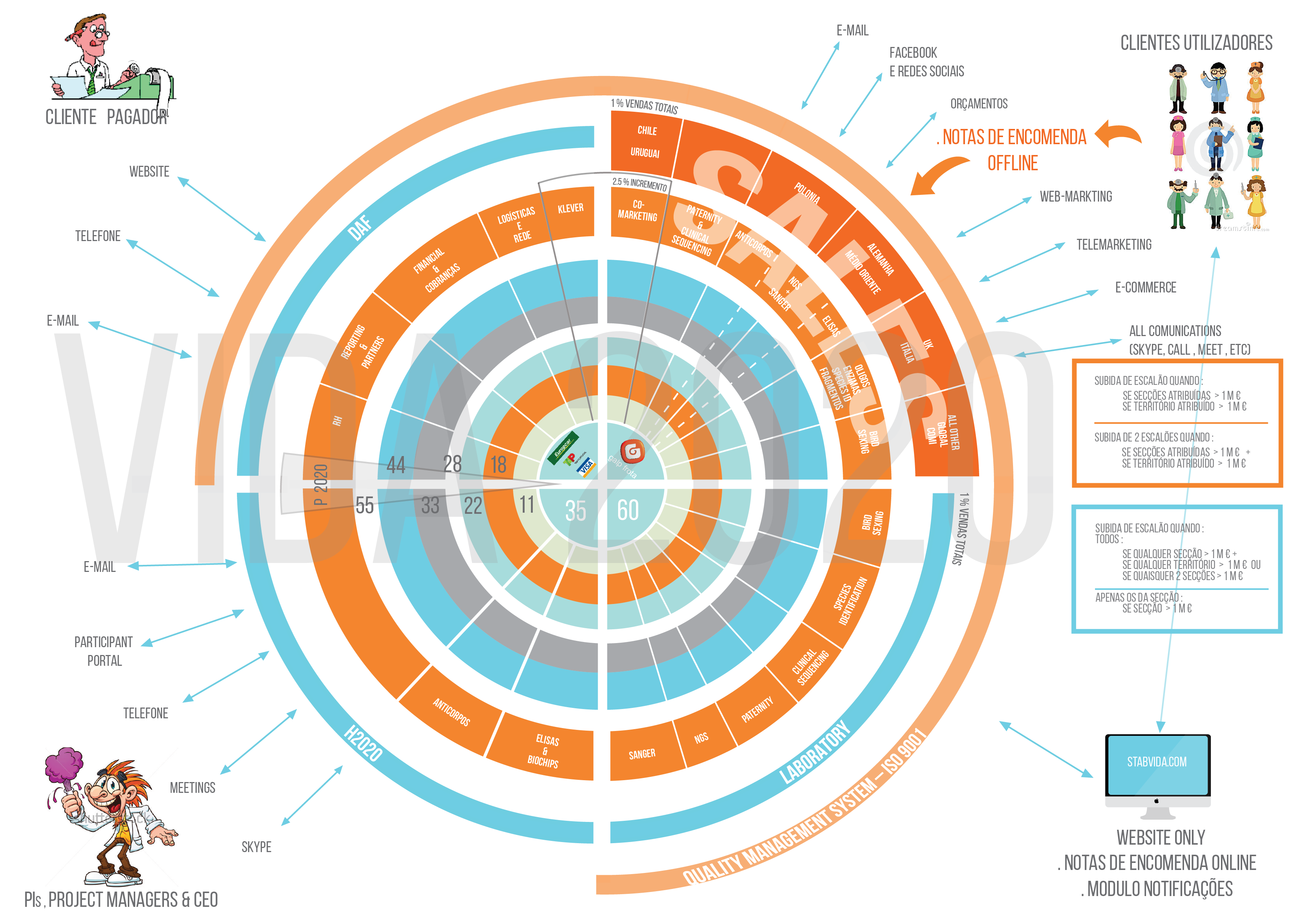
Fund Raising
- WPs
- Leaders
- VC
- Hugo
- H2020
- Orfeu
- P2020
- Doria
Products & Services
- WPs
- Leaders
- Sanger + Fragment
- Daniela
- Oligos Sus/Taq
- Daniela
- Genotyping Species
- Carla
- NGS 1,2,3,4
- Carla
- Klever e-QC
- Hugo
- Elisas
- Doria
- Mab
- Tatiana
- Birds + Inte
- Tatiana

Our mission is to be Your Easy Genetics Laboratory. Whether you are in Lisbon, Porto, Milan, Rio de Janeiro, Madrid, Cordoba, etc., we aim to provide patients, doctors, scientists, veterinary professionals, and students with an easy and secure channel. At STAB VIDA, we aspire to create an efficient flow for your samples, data, and information, supported by laboratories with highly qualified teams, always with the primary goal of delivering the results you need.
The last decades have witnessed the emergence of information and communication technologies; however, the 21st century will be characterized by advances in genetic technologies, paving the way for new products and markets. This sector represents a high-potential business and is of growing interest to governments, which have highlighted it as a strategic priority in the development of research in genomics and proteomics. It is in this challenging environment that STAB VIDA operates, constantly seeking to improve its performance and explore new opportunities.
In a broader context, our mission involves a continuous commitment to innovation and the adoption of new technologies in the field of biotechnology, through research and development, technology transfer, and a suitable commercial approach for products and services prepared for the global market.
CERTIFICATION AREAS
ISO 9001:2015
Performing genetic tests: PCR, nucleic acid sequencing by Sanger and Next Generation Sequencing (NGS).
ISO 13485:2018
Design and production of in vitro diagnostic medical devices: reagents and equipment based on fluorescence techniques.
QUALITY POLICY
The Management of STABVIDA, Lda., aware of the strategic relevance that stakeholders (customers, legal and regulatory entities, suppliers, partners, and company collaborators) have for the life of the company through the services and products it provides, has established its Quality Policy based on the following principles:
Management encourages all employees to apply these principles within the scope of their responsibilities and duties.
Caparica, January 24th, 2024